Omega 7 Oil and Inflammatory Biomarker Study - this one
Published
About the Study
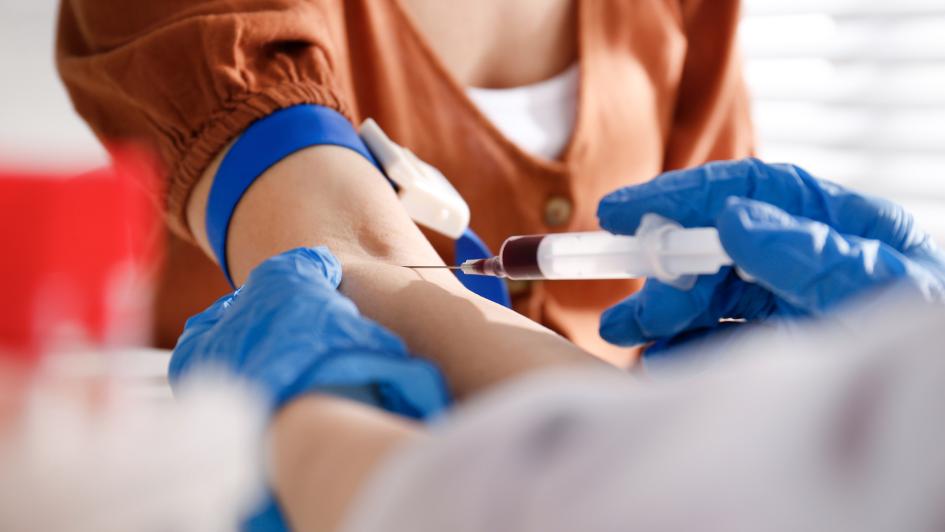
Chronic body pain or discomfort may be associated with chemical inflammatory markers in the blood. We would like to know whether or not three weeks of supplementation with an omega 7 oil can decrease blood inflammatory markers and the level of your discomfort.
The study length is six weeks during which your blood is drawn three times - at the baseline (1st visit), at the end of the third week, and at the end of the sixth week. Each time we draw your blood, you will be compensated $20 and all blood draws will occur at the Bastyr Clinical Research Center in Kenmore, WA. Your baseline blood sample must come back at a specific level to be eligible. You will be notified of eligibility within 72 hours. If you are eligible, you will be given two kinds of supplement bottles in sequence. One of the bottles contains omega-7 oil the, and other contains an inert food-grade oil. The sequence you are given the omega-7 oil is randomly chosen. Each bottle will last for three weeks. You and the clinician do not know which bottle contains omega 7. This process is called, "double blinding".
More specifically, this randomized phase I/II trial studies the side effects of vaccine therapy and trastuzumab with or without a mushroom extract, polysaccharide-K and to see how well it works in treating patients with stage IV human epidermal growth factor receptor 2 (HER2) positive breast cancer. Vaccines made from HER2 intracellular domain (ICD) peptide may help the body build an effective immune response to kill tumor cells that express HER2. Monoclonal antibodies, such as trastuzumab, can block tumor growth in different ways. Some block the ability of tumors to grow and spread. Others find tumor cells and help kill them or carry tumor-killing substances to them. Polysaccharide-K may stimulate the immune system in different ways and stop tumor cells from growing. It is not yet known whether vaccine therapy and trastuzumab are more effective when given with or without polysaccharide-K in treating breast cancer.
Eligibility
Open to adults with chronic pain or discomfort. Chronic refers to pain or discomfort persisting more than three months. The study visits and supplement dispensing take place at Bastyr University in Kenmore Washington. Each study visit will require approximately one hour.
Study Population
We are seeking 50 – 60 people with chronic pain or physical discomfort which may be caused by tissue inflammation.
Inclusion Criteria
- At the baseline, your blood hs-CRP must be higher than 1.0 mg/L
- 18 years old or older
- Have chronic body pain or discomfort which can be caused by tissue inflammation
- Be able to come to Bastyr University (Kenmore, Washington) clinical research center three times
- Stable lifestyle during the study period (six weeks)
- Be able to understand English and comply with study requirements
Exclusion Criteria
- Unable to draw blood
- Documented hyperlipidemia
- Documented cognitive impairment
- Taking anti-hyperlipidemia medications
- Alcohol or other drug dependencies
- Drinking alcohol more than three days a week, three servings or more per occasion
- Possible pregnancy or currently planning to get pregnant
- Breastfeeding
- Objection by his/her primary care clinician or family member
- Take anti-inflammatory or strong pain pharmaceutical medication
- Unable to monitor the dosage of medications
- Unable to communicate in English
- Expecting major lifestyle change during the study period
For more information or to enroll in the study, contact the research team at: researchstudy@bastyr.edu or 425-896-6834.
Please leave your name and contact information and a member of the study team will get back to you shortly.
Project Overview
Status
Active
Study area
Nutrition/Dietary
Other
Principal investigator
Masa Sasagawa, ND, PhD(c)
Project period
September 2017 - December 2018
Completed date (for sorting)
Monday, December 31, 2018
Funded by
Barleans Organic Oil, LLC